By Ana Castro Verde, Ph.D. student and research technician at Computational Clinical Imaging Group, Champalimaud Foundation
Clinical Challenge: Can radical prostatectomy patients help promote a biological validation of radiomics signatures?
Prostate cancer patients with less aggressive tumors would benefit from being in active surveillance instead of undergoing treatment with potential complications. Magnetic resonance imaging (MRI) offers high sensitivity to diagnose prostate tumors but is limited by variability in the radiologists’ interpretations. With the advent of Artificial Intelligence (AI) in medical imaging it is possible to improve patient stratification and to guide the best treatment options. A sub-field of AI, the so-called radiomics, treats images as mineable data by extracting features that are not observable through the human eye. For a clinical implementation of radiomics, it is missing a causal relationship between radiomics features and clinical outcomes. To promote the usage of AI models in clinical ractice, one of the main challenges in the field is to perform a biological validation of the radiomics features using the biopsy ground truth. Dr. Nikolas Papanikolaou, Principal Investigator in Oncologic Imaging at the Fundação Champalimaud, is leading the fourth clinical ProCAncer-I use case (UC4) on the Radiologic – Histopathologic correlation to provide biology-based validation of AI models. Patients undergoing radical prostatectomy offer an opportunity to correlate radiomics features with the ground truth from the prostate specimen. Prostate segmentations are automatically generated using Deep Learning algorithms developed within the Computational Clinical Imaging Group and validated by a radiologist from the Radiology Department at our institution. These segmentations are used for the creation of patient-specific 3D-printed molds developed in collaboration with the Hardware and Software Platform (Figure 1). The patient-specific molds allow the histopathology technicians to cut the prostate specimen with the same spacing as the MRI slices.

Figure 1: MRI-based patient-specific 3D-printed prostate molds
.
Afterwards, Python scripts are developed for stitching of prostate tissue quadrants to reconstruct a pseudo-whole-mount histopathology prostate section and for rad-path registration by registering histopathology sections to the corresponding MRI slides (Figure 2). These algorithms, guided by the usage of patient-specific molds, have shown promising results to promote a direct correspondence between both imaging modalities. Extracted radiomics features will be correlated with the biological ground truth. One potential application of this work is the detection of invisible lesions in MRI, only observable through the analysis of biological tissue. The next steps include the prospective acquisition of more than 80 prostatectomy patients to validate our algorithms and to eventually join efforts with other ProCAncer-I clinical partners – La Fe University and Polytechnic Hospital in Spain and Istituto di Candiolo – Fondazione del Piemonte per l’Oncologia in Italy – to increase the sample size. In the close future, we expect to be able to prevent unnecessary surgeries by using solely imaging characteristics to promote early cancer detection.
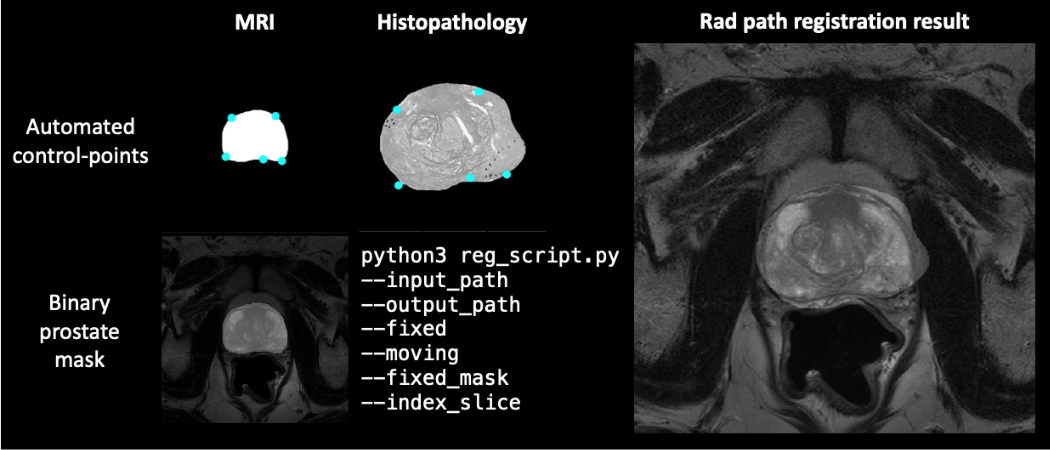
Figure 2: Rad-path registration result for corresponding images, guided by automated control points and binary prostate mask

