By Joao Correia, Innovation Manager at Biotronics3D Limited (B3D)
The ProCAncer-I eCRF (electronic case report form) and Data Upload Tools support ProCAncer-I clinical partners in the process of compiling the required information and follow the defined protocols for uploading data to the project’s cloud repositories. The ProCAncer-I eCRF is a set of digital forms used to collect the clinical data of the patients that are involved in the project research. The forms were designed iteratively with the Clinical Partners and the AI developers to collect specific information for 9 Use Cases defined in the project.
Aligned with the anonymisation strategy, the tools were designed to be used for data collection locally, inside the networks of the clinical partners, and execute a white-list anonymisation step before uploading to the ProstateNET cloud repositories. The tool integrates with the RSNA CTP anonymisation tool and with the ProCAncer-I repository services such as authentication, DICOM API Gateway and Metadata Catalog API, enabling the anonymisation and upload of data using methods that comply with data protection, privacy and security requirements. The tool is designed to follow a 5 steps workflow from the selection of the folder containing the DICOM files, to the anonymisation, edition of the clinical information and upload of DICOM and clinical information data.
The ProCAncer-I eCRF and Data Upload Tool, shown in Figure 1, is a windows application that should be installed on Windows 10 or above computers with internet connection. The tool also has a command line version, the eCRF_Batch, that allows bulk upload of data for Clinical Partners that can programmatically retrieve the clinical data and generate the specific json files.
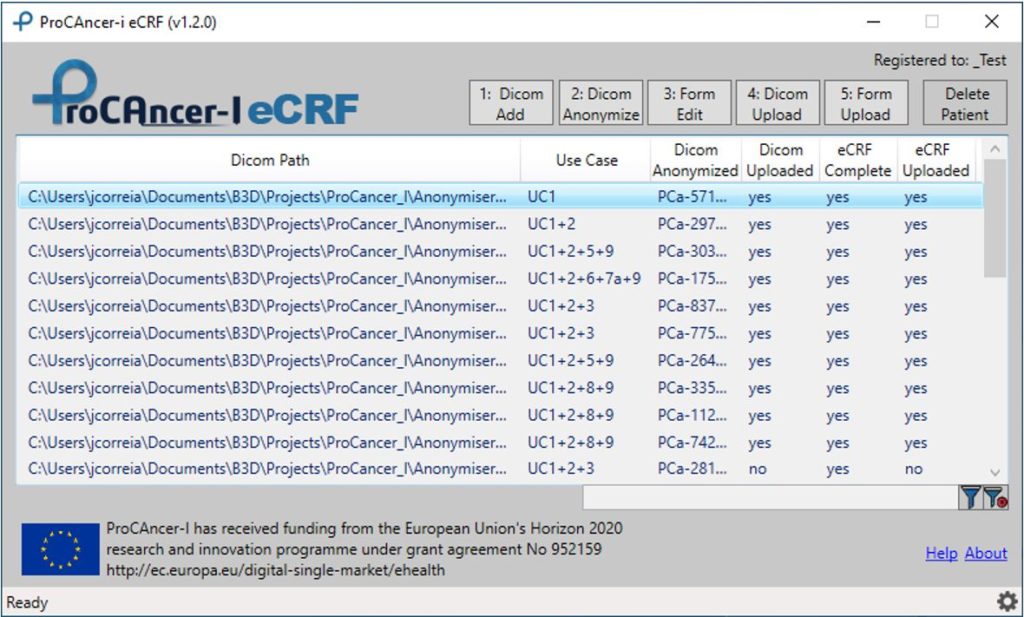
Figure 1. eCRF and Data Upload Tool main window
The workflow starts with “1: DICOM Add” for the selection of a case folder which contains the DICOM studies for the patient. The second step “2: DICOM anonymise” runs the CTP anonymisation tool with the pre-defined anonymisation script that completely anonymises the DICOM files as defined in the project. The third step “3: Form Edit” allows the user to select for each patient a particular form according to the available clinical, imaging, pathology, treatment, follow-up information. Since a single patient could be useful in more than one single Use Case, we optimized data collection in the eCRF, to avoid duplicates and time-consuming data entry by clinical partners. The fourth step “4: DICOM upload” allows to upload the anonymised DICOM files of the selected case. The final step in the workflow is “5: Form Upload” that allows to upload the clinical information of the selected case to the ProCAncer-I cloud repository. The eCRF Forms enable users to select different Forms according to the imaging and non-imaging data available for each patient.
Each Form contains different sections to be filled with clinical, imaging, pathology, treatment and follow-up information. Each tab will contribute to form a specific subgroup of patients which will be used to create the final dataset for one or more Use Cases. In Figure 2, two forms are depicted for Use Cases 1 and 2.
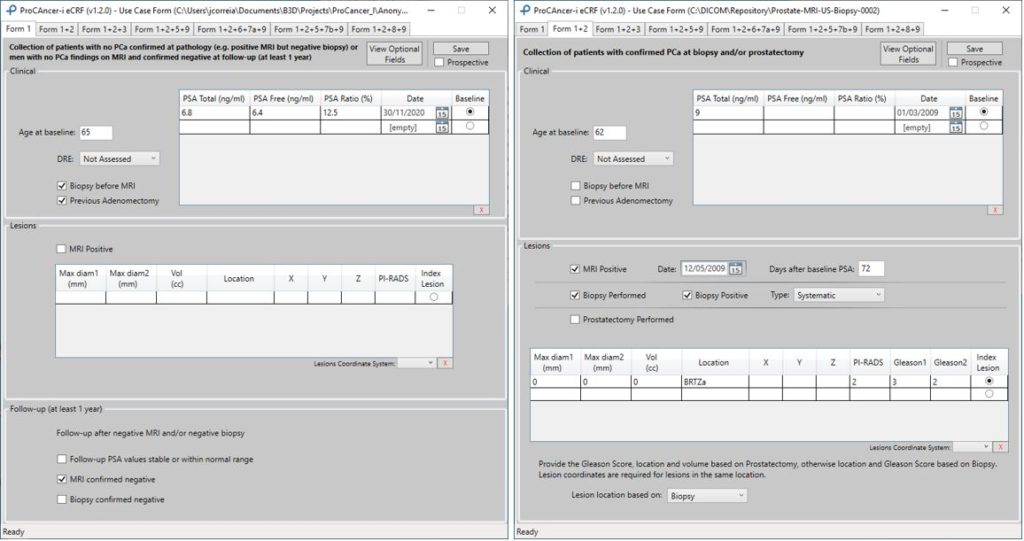
Figure 2. eCRF forms for Use Case 1 and 2
The eCRF batch mode is intended to support partners contributing a large volume of data to upload, who have already a database with the clinical information and also have the means to transform the clinical data into the defined JSON formats. The batch mode provides a standard way for partners to implement their mechanism to export existing DICOM and clinical data and metadata to the eCRF format. Partners are required to extract the information in their current format (e.g., spreadsheet and databases) using their preferred scripting/programming language and generate JSON files along with a copy of all the DICOM data following a defined directory structure.
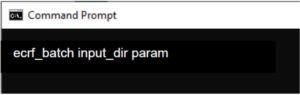
Figure 3,the eCRF Batch
As shown in Figure 3, the eCRF Batch is a Windows based binary program that reads the corresponding files from the folders specified in an input directory, verify their correctness and upload them without the need of the current workflow-guided eCRF version. DICOM data is anonymized using the CTP anonymisation tool from RSNA, following the whitelist script designed and provided by the ProCAncer-I project. Besides several design iterations of the eCRF to reach a balance between data requirements, data availability and data collection effort, the need to comply with different network architectures and restrictions led to several updates of the data upload tools. Up to November 2022, using the eCRF and Data Upload tools, the Clinical Partners of ProCAncer-I project have uploaded to the ProstateNET cloud repositories +8,000 cases, corresponding to +16,000 data points, containing almost 5 million images.

