Objectives
The vision of the ProCAncer-I project is to be realized by a set of specific, interrelated, ambitious, yet totally realistic, objectives. Realization of these objectives will enable us to address crucial questions related to prostate cancer management through the disease continuum on the one hand and to deliver a novel infrastructure enabling experimentation of AI-based solutions to improve diagnosis, treatment and follow-up and contribute to more precise and personalised management of cancer, on the other.
Develop a comprehensive data resource related to prostate cancer for clinical care, research and innovation.
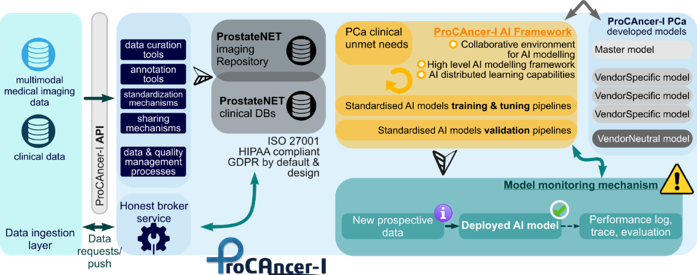
ProCAncer-I will create a vast, diversified and multidisciplinary repository, fed by a large collection of multi-parametric MRI scans (mpMRI) which is the recommended technique in prostate cancer imaging including a high-resolution T2 weighted-imaging and at least two physiology-based MRI techniques12.. The participating clinical partners will congregate mpMRI and clinical data, retrospectively and prospectively, from more than 17.000 PCa patients (6000 prospective mpMRI cases), including baseline examinations and follow up studies to form the ProstateNET dataset counting more than 1.5 million image representations of prostate cancer.
Design, develop and deploy the (security, interoperability, transparency, sharing) for a medical imaging repository and cloud-based platform compliant to necessary standards trustworthy AI analytics workflows.
ProCAncer-I will establish a cooperative framework for seamless data and knowledge sharing among researchers, clinicians and AI developers through a number of web APIs, data harmonization and indexing modules, a metadata catalogue driven by advanced ontologies and a semantic search engine for integrated end-user workflow supported by cooperative annotation tools for regions of interest (ROIs) and for collaborative extensions of underlying data models. The project will deliver a state-of-the-art scalable imaging repository based on the cloud-based infrastructure. additionally, the ProCAncer-I will be an AI on-Demand Platform that supports an AI ecosystem and share AI resources for the development, implementation, verification and validation of the advanced AI models related to PCa. In addition, special efforts will be devoted to making sure that seamless interoperability with other relevant European infrastructures will be achieved.
Develop and deploy novel AI models to address the unanswered clinical questions regarding prostate cancer management across the disease continuum.
ProCAncer-I will exploit state of the art AI techniques (Radiomics and Deep Learning) to develop AI validated models for a range of pressing clinical scenarios and questions that include: 1) Accurate detection of prostate cancer both in the peripheral as well as the transitional zone; 2) Characterization of cancer according to its biological aggressiveness into clinically significant and non-significant disease; 3) Identification of patients with metastatic prostate cancer as early as possible; 4) Radiologic – Histopathologic correlation to provide biology-based validation of AI models; 5) Prediction of the risk of disease recurrence; 6) Prediction of treatment response in case of Radiation therapy; 7) Prediction of post radical prostatectomy and/or Radiation-induced urinary toxicity; 8) AI-powered patient stratification for enrolment in active surveillance programs.
Create a sustainable infrastructure for future use and develop novel sustainability models.
ProCAncer-I aims to adjust current cloud-based certified infrastructures for medical data hosting for the specific needs of prostate cancer decision making by adding tools and utilities that are related to preprocessing, segmentation, annotation, ontology and AI models. ProCAncer_I’s infrastructure will remain open after the end of the project with the goal to be widely used by the PCa research community for developing and validating new AI models addressing clinical questions in PCa, in a controlled manner To this end, ProCAncer will address all necessary cancer-related regulatory issues that will facilitate AI model validation/acceptance and promote their commercial exploitation, therefore, ensuring that both clinical researchers and companies will be highly interested to keep using the platform after the competition of the project.
Validate, verify and explain or interpret the performance of AI models in order to increase trust and render them applicable in clinical practice.
ProCAncer-I will gather the largest image database worldwide of PCa retrospective data in order to address the heterogeneity in pathophysiology as well as the acquisition process, which has been a long standing obstacle for any process of harmonization. The proposed multi-center, three-phase AI modeling methodology comprising a master-global model, vendor-specific models and vendor-neutral models that will be introduced in this project combined with the large volume of congregated data will increase trust level by accurately measuring the performance of AI models and comparing it to previously published human or machine performance.
Advance clinical knowledge and patient care in the second most common form of cancer.
ProCAncer-I will facilitate the development and validation of new AI tools models responding to specific clinical questions via computer-assisted diagnosis (CAD), and also support clinical consults with external second-opinion workflows. By supporting rapid validation, benchmarking and market release, ProCAncer-I will contribute to improving the detectability of prostate cancer, accuracy differentiation of clinically significant from non-significant disease, of diagnosis, prognostic prediction of disease recurrence, assessment of treatment-related toxicity and accurate patient stratification, minimizing medical errors, enhancing clinical outcomes and quality of care.
Addressing a significant societal problem in the second most common form of cancer ( Prostate Cancer) by supporting efficient disease management across the care continuum.
In ProCAncer-I, we plan to devote significant efforts towards establishing the ProCAncer-I regulatory framework which will provide guidance towards the validation/qualification of ProCAncer-I AI clinical tools. The framework will consolidate the input from scientific experts and health authorities, and will take into consideration any published relevant guidelines e.g., from the High-Level Expert Group on Artificial Intelligence; from the Medical Device Coordination Group and relevant implementing EU legislation; and from international regulations. GDPR compliance analysis, ethics issues and analysis of legal requirements in terms of de-identification of patients’ data when processing for AI-based tool development purposes, and assessments for building the ProCAncer-I honest broker mechanism will also take place.




